Abstract
BCL2 is an antiapoptotic protein commonly expressed in hematologic malignancies. Overexpression of BCL-2 is a poor prognostic factor in acute myeloid leukemia (AML). Venetoclax (ABT-199) is a highly selective BCL2 inhibitor that can induce cell death in multiple leukemia cell lines. Recently, venetoclax received an FDA breakthrough therapy designation for use in combination with hypomethylating agents in treatment-naïve patients with AML who are unfit for intensive chemotherapy. However, venetoclax was only modestly effective as monotherapy in relapsed/refractory AML (19% CR/CRi). The aim of the current study is to integrate genomic and functional screen data to identify biomarkers to predict venetoclax sensitivity and resistance in AML, and to identify potential venetoclax combination treatment strategies.
In this study, we investigated approximately 200 AML patient samples and correlated clinical parameters, whole exome sequence data, and RNAseq gene expression data with in vitro drug screening data (drug area under the curve (AUC)) to identify subsets of AML samples with sensitivity or resistance to venetoclax alone and in combinations with 10 small molecular inhibitors (Array-382, dasatinib, JQ-1, idelalisib, quizartinib, palbociclib, panobinostat, ruxolitinib, sorafenib, and trametinib).
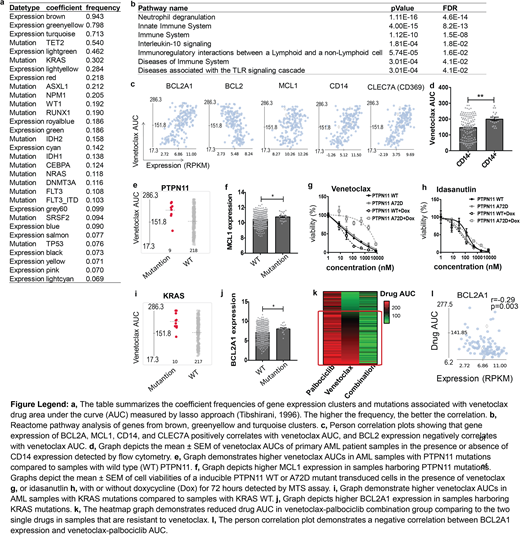
For gene expression, we observed that venetoclax correlated with 3 gene expression clusters (coefficient frequency: 0.94, 0.80 and 0.71 respectively) among 21 gene expression clusters in AML, associated with innate immune system, neutrophil degranulation, and interleukin-10 signaling. Among the BCL2 gene family, venetoclax AUC positively correlated with BCL2A1 (r=0.59, p<0.0001) and MCL1 (r=0.26, p=0.001) expression, whereas it negatively correlated with BCL2 (r=-0.53, p<0.0001) expression. BCL2A1 is the only BCL2 family gene within all three clusters and correlated the best with venetoclax sensitivity. Interestingly, within the three gene clusters, we observed that cell surface markers CLEC7A (CD369) and CD14 correlated with venetoclax sensitivity (r=0.68 and 0.64, p<0.0001). AML patient samples expressing CD14 detected by flow cytometry also demonstrated reduced venetoclax sensitivity (p=0.005), which could potentially serve as a biomarker to identify venetoclax resistant patients. For cytogenetic categories and mutations, we observed that AML samples harboring PML-RARA translocations, WT1, and FLT3 with IDH1 mutations are more sensitive to venetoclax, and samples with TET2, KRAS, PTPN11 and SF3B1 mutations are more resistant. We validated the effect of WT1, KRAS, and PTPN11 mutations on venetoclax sensitivity by performing drug assays on mouse bone marrow stem cells and/or AML cell lines overexpressing each mutant. Samples harboring PTPN11 mutations demonstrated high MCL1 expression, and PTPN11 mutant-transduced cells remain sensitive to Idasanutlin, which was previously shown to downregulate MCL1 expression. Samples with KRAS mutations demonstrated high BCL2A1 expression, which potentially mediate venetoclax resistance. For venetoclax drug combinations, we observed that venetoclax-trametinib demonstrated a synergistic effect on samples that are sensitive to venetoclax, whereas venetoclax-palbociclib, venetoclax-Array-382, venetoclax-sorafenib, venetoclax-ruxolitinib, venetoclax-dasatinib, and venetoclax-idelalisib are active against samples that are resistant to venetoclax, indicating potential therapeutic combinations. Interestingly, the CDK inhibitor palbociclib demonstrated no effect on the majority of AML samples and does not correlate with the BCL2A1 expression as a single agent, yet shows the most robust synergy with venetoclax, especially on samples that are resistant to venetoclax and with high BCL2A1 expression, indicating a potential synthetic lethal interaction. Venetoclax-palbociclib AUC also negatively correlated with CLEC7A and CD14 expression, indicating that venetoclax-palbociclib could circumvent venetoclax resistance to treat patients with high CLEC7A and/or CD14 expression.
In summary, we have identified that CD14 and/or CLEC7A could be used as biomarkers to predict venetoclax sensitivity in AML, and we propose to combine venetoclax and palbociclib to treat patients with a venetoclax resistant profile (high CD14/CLEC7A expression or high BCL2A1 expression, or presence of KRAS mutations).
Druker:Beta Cat: Membership on an entity's Board of Directors or advisory committees; Fred Hutchinson Cancer Research Center: Research Funding; McGraw Hill: Patents & Royalties; Vivid Biosciences: Membership on an entity's Board of Directors or advisory committees; Oregon Health & Science University: Patents & Royalties; Bristol-Meyers Squibb: Research Funding; GRAIL: Consultancy, Membership on an entity's Board of Directors or advisory committees; ARIAD: Research Funding; Third Coast Therapeutics: Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Consultancy, Membership on an entity's Board of Directors or advisory committees; Monojul: Consultancy; Novartis Pharmaceuticals: Research Funding; Millipore: Patents & Royalties; Leukemia & Lymphoma Society: Membership on an entity's Board of Directors or advisory committees, Research Funding; ALLCRON: Consultancy, Membership on an entity's Board of Directors or advisory committees; Aileron Therapeutics: Consultancy; Patient True Talk: Consultancy; Cepheid: Consultancy, Membership on an entity's Board of Directors or advisory committees; Henry Stewart Talks: Patents & Royalties; Celgene: Consultancy; Amgen: Membership on an entity's Board of Directors or advisory committees; MolecularMD: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Aptose Therapeutics: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Majeti:BioMarin: Consultancy; Forty Seven, Inc: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Tyner:Incyte: Research Funding; AstraZeneca: Research Funding; Janssen: Research Funding; Takeda: Research Funding; Leap Oncology: Equity Ownership; Seattle Genetics: Research Funding; Genentech: Research Funding; Gilead: Research Funding; Syros: Research Funding; Aptose: Research Funding; Agios: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal